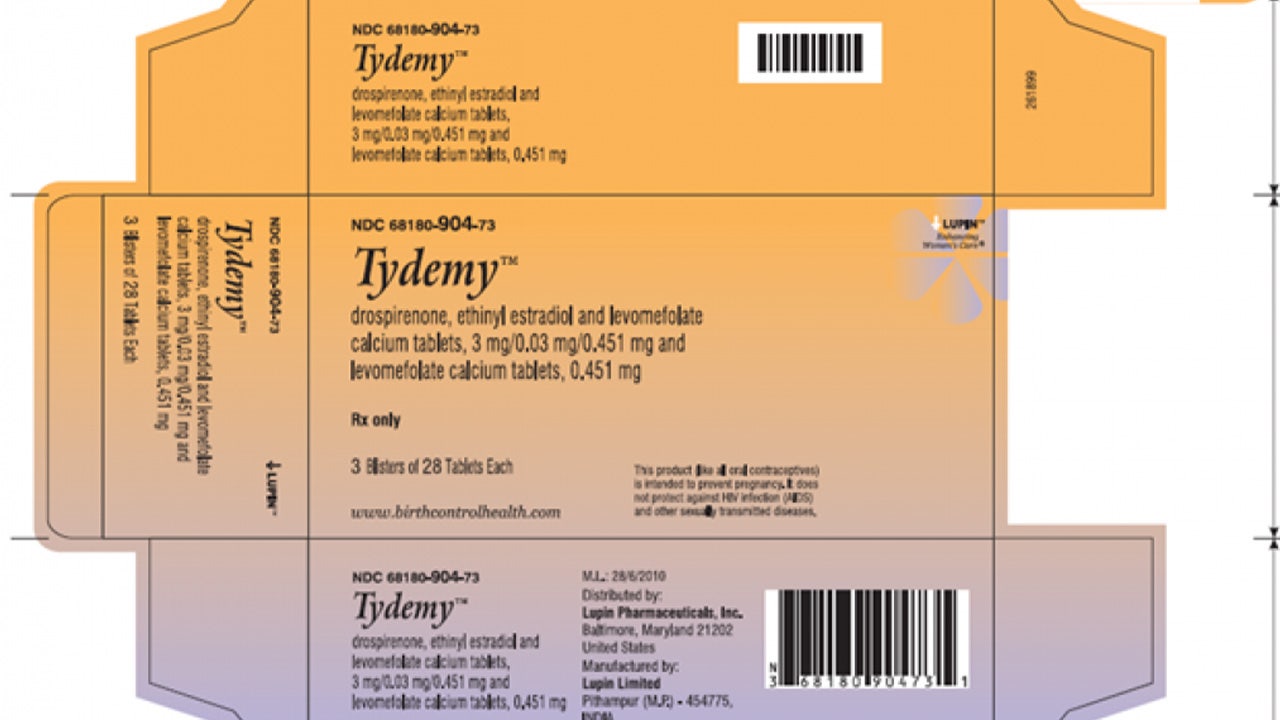
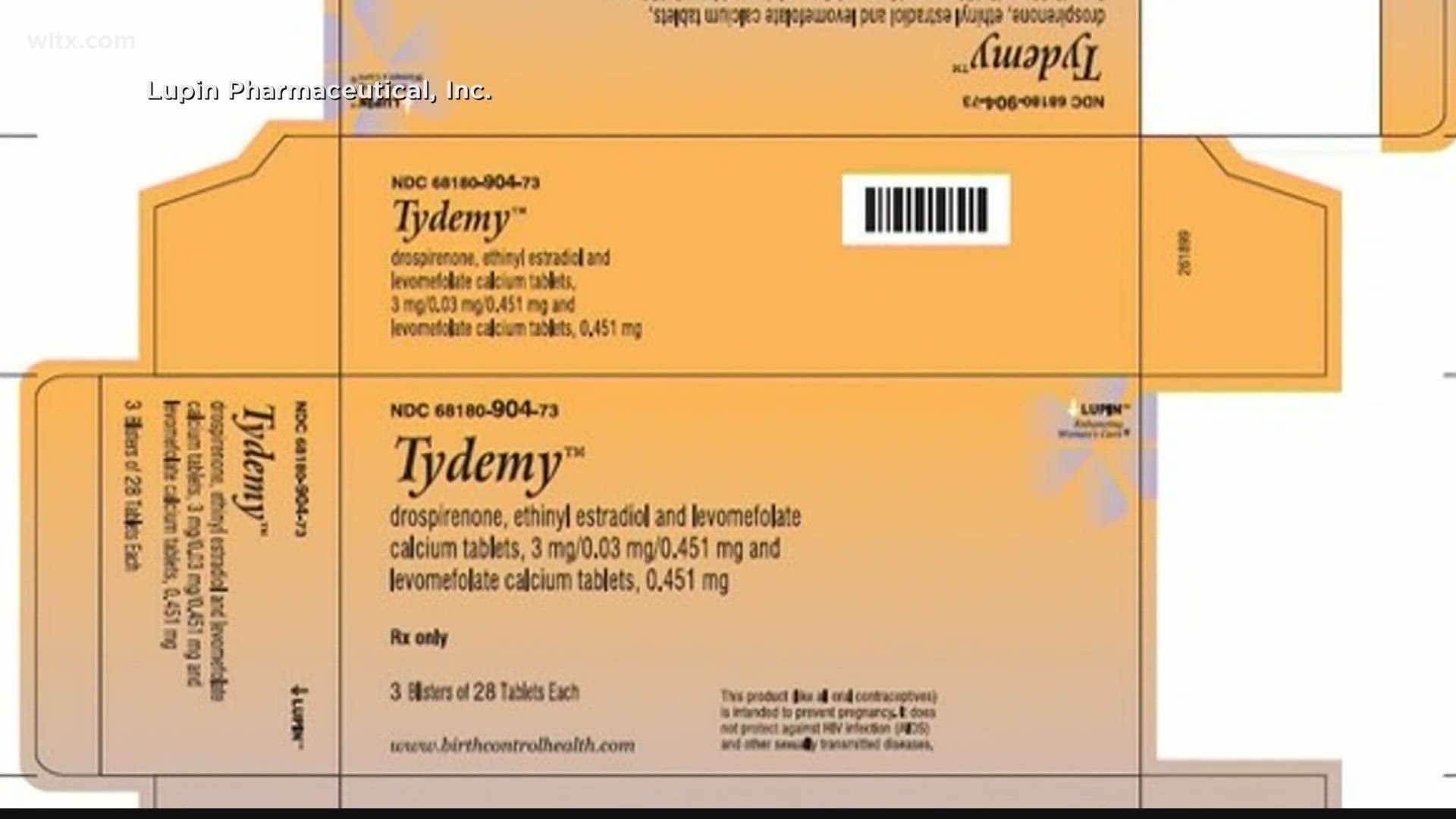
Tydemy oral birth control recalled, FDA warns that reduced efficacy may result in unexpected pregnancies | Fox News

Levonorgestrel and Ethinyl Estradiol Tablets USP (0.1 mg/0.02 mg) and Ethinyl Estradiol Tablets USP (0.01 mg) - Lupin Pharmaceuticals, Inc.

Birth control pills voluntarily recalled as FDA warns they may have 'reduced effectiveness' - ABC News

These highlights do not include all the information needed to use NORGESTIMATE AND ETHINYL ESTRADIOL TABLETS safely and effectively. See full prescribing information for NORGESTIMATE AND ETHINYL ESTRADIOL TABLETS.NORGESTIMATE AND ETHINYL ESTRADIOL




/cloudfront-us-east-1.images.arcpublishing.com/gray/5N53UXABKBJUVFMOUF77GQIO4Y.jpg)











/cloudfront-us-east-1.images.arcpublishing.com/gray/DFKXUJAABRPI3AKOECN6POKDJI.jpg)
/cloudfront-us-east-1.images.arcpublishing.com/gray/F7KESGCSEVOSPOD5UPDYYYNW5U.jpg)